
Cytokine-mediated communication: a quantitative appraisal of immune complexity.
#Copay assistance interferon gamma 1b trial#
Phase II clinical trial of intratumoral application of TG1042 (adenovirus-interferon-gamma) in patients with advanced cutaneous T-cell lymphomas and multilesional cutaneous B-cell lymphomas. Human platelets possess receptors for a lymphokine: demonstration of high specific receptors for HuIFN-gamma. Modeling the receptor pharmacology, pharmacokinetics, and pharmacodynamics of NKTR-214, a kinetically-controlled interleukin-2 (IL2) receptor agonist for cancer immunotherapy. Prolonged circulation half-life of interferon γ activity by gene delivery of interferon γ-serum albumin fusion protein in mice. Three-dimensional structure of recombinant human interferon-gamma. The dual role of mir-146a in metastasis and disease progression. Diverse roles of miR-29 in cancer (review). MicroRNA-142-3p inhibits IFN-γ production via targeting of RICTOR in Aspergillus fumigatus activated CD4+ T cells. Regulation of human natural killer cell IFN-γ production by microRNA-146a via targeting the NF-κB signaling pathway. MicroRNA-29 regulates T-box transcription factors and interferon-γ production in helper T cells. Regulation of nuclear gamma interferon gene expression by interleukin 12 (IL-12) and IL-2 represents a novel form of posttranscriptional control. Stabilization of IFN-gamma mRNA by MAPK p38 in IL-12- and IL-18-stimulated human NK cells.
#Copay assistance interferon gamma 1b Activator#
Synergy of IL-12 and IL-18 for IFN-gamma gene expression: IL-12-induced STAT4 contributes to IFN-gamma promoter activation by up-regulating the binding activity of IL-18-induced activator protein 1. The authors cloned IL-18 (also known as IGIF) and showed in vitro induction of IFNγ with recombinant IL-18 via an IL-12-independent pathway. Cloning of a new cytokine that induces IFN-gamma production by T cells. A comprehensive pathway map of IL-18-mediated signalling. IL-12 family cytokines: immunological playmakers. Second messenger role of arachidonic acid and its metabolites in interferon-gamma production. Interleukin 2-mediated immune interferon (IFN-gamma) production by human T cells and T cell subsets. Leukotrienes: positive signals for regulation of gamma-interferon production. A mechanism underlying STAT4-mediated up-regulation of IFN-gamma induction in TCR-triggered T cells. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Regulation of interferon-gamma gene expression by nuclear factor of activated T cells.

Evolutionarily conserved sequence elements that positively regulate IFN-gamma expression in T cells. The NeST long ncRNA controls microbial susceptibility and epigenetic activation of the interferon-γ locus. Cutting edge: changes in histone acetylation at the IL-4 and IFN-gamma loci accompany Th1/Th2 differentiation.
Cutting edge: distal regulatory elements are required to achieve selective expression of IFN-gamma in Th1/Tc1 effector cells. Expression of transfected human interferon-gamma DNA: evidence for cell-specific regulation.

Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Interferon-like virus-inhibitor induced in human leukocytes by phytohemagglutinin.
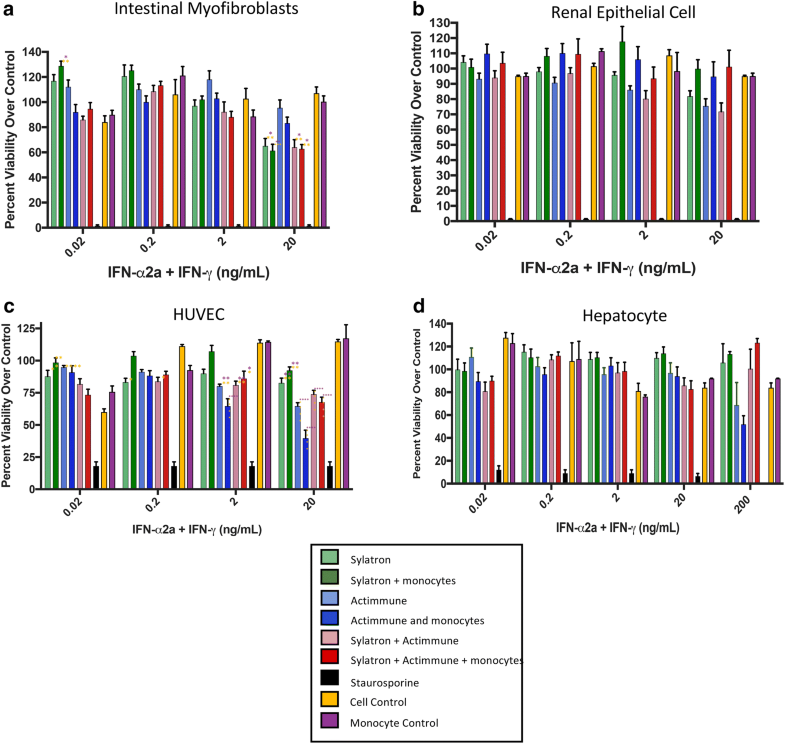
Here, we review the cells that produce IFNγ and the different effects of IFNγ in the tumour microenvironment, highlighting the pleiotropic nature of this multifunctional and abundant cytokine. Given that all nucleated cells can respond to IFNγ, the functional consequences of IFNγ production need to be carefully dissected on a cell-by-cell basis. Patients resistant to these therapies commonly have molecular aberrations in the IFNγ signalling pathway or express resistance molecules driven by IFNγ. Many cancer immunotherapies and chemotherapies induce IFNγ production by various cell types, including activated T cells and natural killer cells. Interferon-γ (IFNγ) is a cytokine that has both protumour and antitumour activities, suggesting that it may serve as a nexus for responsiveness to immunotherapy. However, many patients do not respond to immunotherapy, galvanizing the field to define the mechanisms of pre-existing and acquired resistance. Cancer immunotherapy offers substantive benefit to patients with various tumour types, in some cases leading to complete tumour clearance.


 0 kommentar(er)
0 kommentar(er)
